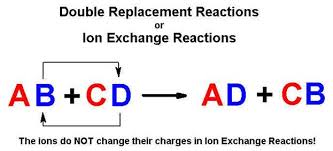
A precipitate is expected when an aqueous solution of potassium iodide is added to an aqueous solution of. A B-C A-C B.

Double Displacement Reaction Definition Examples Video Lesson Transcript Study Com
Write the balanced chemical equation the.
. Chemistry Chemical Reactions Single Replacement Reactions. In a single-displacement reaction one element displaces another element in a compound. Sodium sulfate ironII chloride calcium perchlorate barium hydroxide lead nitrate.
CopperII chloride and leadII nitrate react in aqueous solutions by double replacement. The sulfuric acid reacts with the sugar. The sulfuric acid forms new products.
The sulfuric acid is not consumed or react with the reactant. The sulfuric acid is not consumed or react with the reactant. 2015 AP Chemistry free response 3a Science Chemistry library Chemical reactions and stoichiometry Types of chemical reactions Double replacement reactions.
Is the concentrated H2SO4 a catalyst in this reaction. The general equation for a single-displacement reaction is. A and B must be either different metals including H or different halogens.
What happens in a single displacement reaction. C12H22O11 11 H2SO4 12 C 11 H2SO4 11 H2O How do you know the concentrated sulfuric acid is a catalyst.

Double Replacement Reaction Definition And Examples

Chemical Reactions 1 Of 11 Double Replacement Reactions An Explanation Youtube

Double Replacement Double Displacement Reaction

Introduction To Double Replacement Reactions Youtube
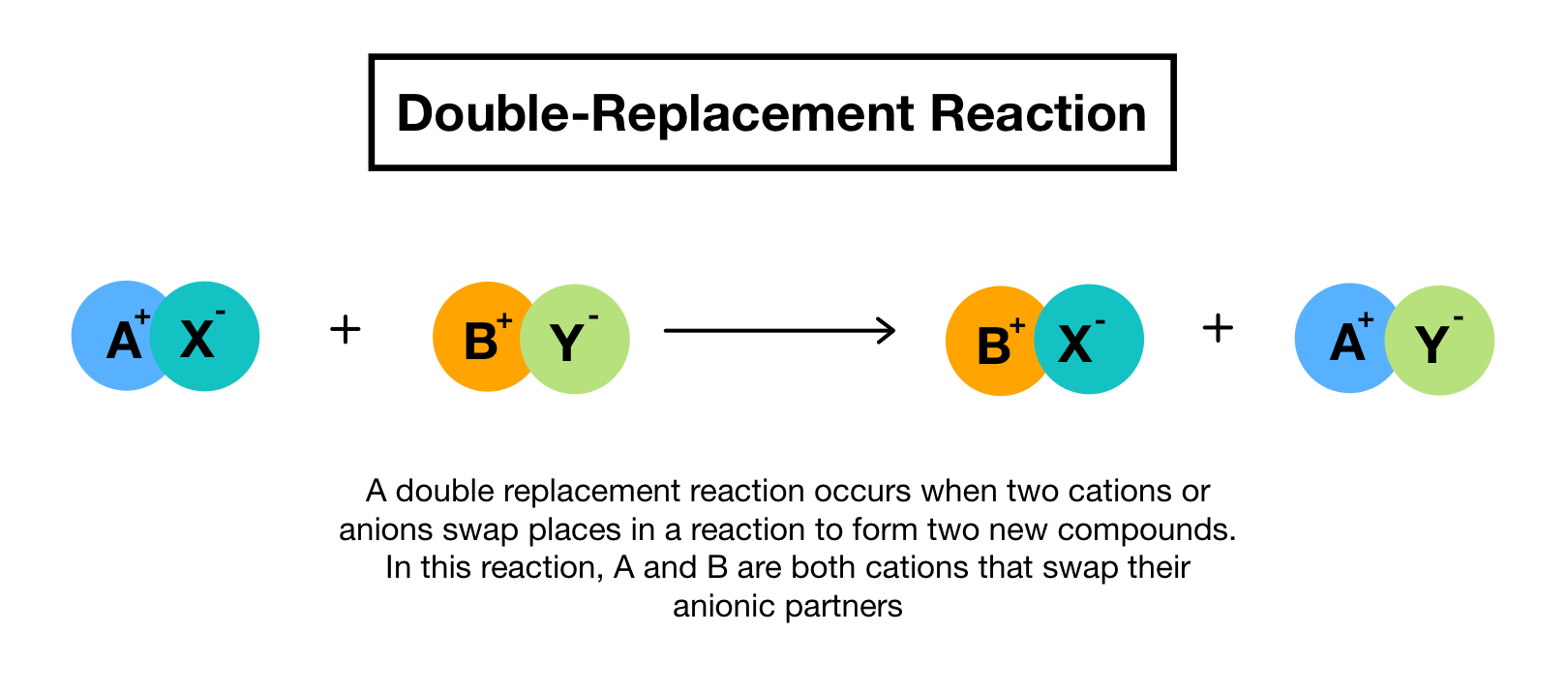
Double Replacement Reactions Definition Examples Expii

Chemical Reactions 2 Of 11 Single Replacement Reactions An Explanation Youtube

0 comments
Post a Comment